light transmission of carbon element
Also they are used in the manufacture of ink and paints. Carbon is characterized as the 4 th most abundant element in the universe and the 15 th most abundant element in the Earths crust.

Introduction To Carbon Sciencedirect
5-15Attenuation of light increases greatly however if the ice is stained with dissolved organic matter or is cloudy that is contains air bubbles or forms irregular crystals upon freezing Adams 1978 1981Absorption is greatest in the red portion of the.
. Plant carbon isotopic compositions are controlled by atmospheric CO 2 and the supply and demand of CO 2 in photosynthesis the process used by plants to convert light energy from the sun into chemical energy. 6 P o u t T T P L P L 1 R T 1 η e A 1 D where P out includes both light that was scattered by the polymer and light that came directly from the laser source. We show that FE is valuable for describing the far-field transmission of light in optical systems.
It used for a wide range of applications including the manufacture of car batteries and dry cells. The light wave could be absorbed by the object in which case its energy is converted to heat. Carbo coal is a chemical element with the symbol C and atomic number 6.
Three isotopes occur naturally 12 C and 13 C being stable while 14 C is a. Carbon is most commonly obtained from coal deposits although it usually must be processed into a form suitable for commercial use. In plant cells the Calvin cycle is located in the chloroplasts.
We have previously learned that visible light waves consist of a continuous range of wavelengths or frequencies. It is readily soluble in water or carbon disulfide forming a red solution is less. Carbon is the majority element in charcoal.
Atmospheric carbon dioxide allows visible light in but prevents some infrared escaping the natural greenhouse effect. Three naturally occurring allotropes of carbon are known to exist. Lithium peroxide Li 2 O 2 in presence of moisture not only reacts with carbon dioxide to form lithium carbonate but also releases oxygen.
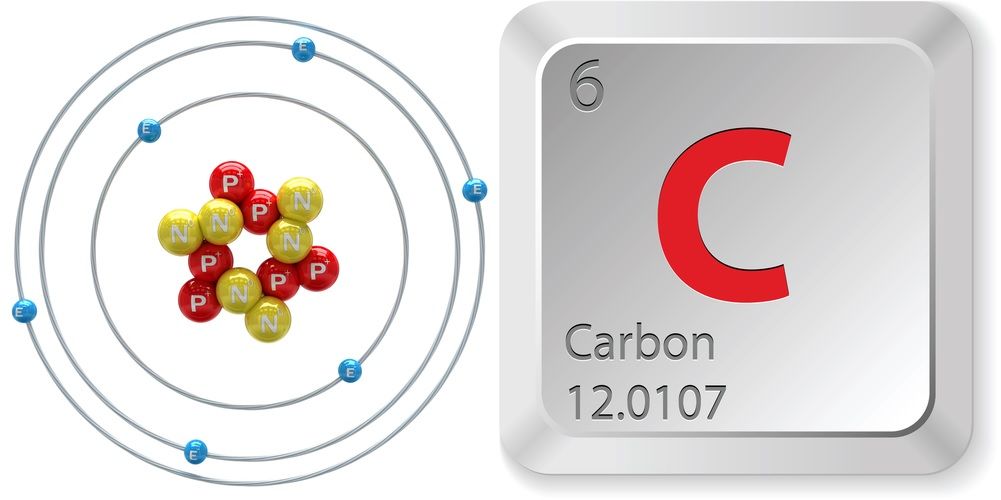
In 2013 as a result of combusting fossil fuels with oxygen there was 390 ppm. The nuclear resonance or energy levels for helium beryllium carbon and oxygen. It is nonmetallic and tetravalentmaking four electrons available to form covalent chemical bondsIt belongs to group 14 of the periodic table.
It can be seen that the transmission of the CNT on the glass substrate with ultrathin Al 2 O 3 and ZnO coatings in the visible light range is improved in comparison with that without coatings but the transmission of the CNT coated with the metal Au is reduced. Light Absorption Reflection and Transmission. Open grown plants are grown in an area that is well ventilated and receives natural CO 2.
Light reactions harness energy from the sun to produce chemical bonds ATP and NADPH. This then allows the total laser radiation power output P out after passing through the polymer thickness D to be calculated from. Amorphous carbon is formed when a material containing carbon is burned without enough oxygen for it to burn completely.
One way to empirically analyze the extremely high light harmonics that produce matter is by carefully studying the atomic absorption and emission spectra for the known elements. The reaction is as follows. This transmission can be.
These energy-carrying molecules are made in the stroma where carbon fixation takes place. When a light wave with a single frequency strikes an object a number of things could happen. The ability to transmit light is another convenient property used in the identification of minerals.
The elements of the Periodic Table are also an artifact of consciousness of light. Fano-resonance analysis abstract The Fanos equation FE describes electron transition from the ground state to the excited state. In other terms carbon makes up matter.
This keeps the Earth warm enough to sustain life. Atomic Weight average mass of the atom. Title Light transmission in carbon nanotube array.
Therefore it is used as fuel. T T is the total transmittance. Each spectra provides a unique lower harmonic light signature for each element.
Atomic Symbol on the Periodic Table of Elements. Another carbon form the graphite is used for high temperature crucibles dry cell and light arch electrodes for pencil tips and as a lubricant. But cosmic mass density is not the only thing that must have been exquisitely fine-tuned for the universe to contain any carbon.
Carbon makes up only about 0025 percent of Earths crust. And it turns out that the reaction that creates carbon from elements lighter than carbon is fine-tuned to an amazing degree. Opaque minerals such as.
Transmission of light is the moving of electromagnetic waves whether visible light radio waves ultraviolet etc through a material. Carbo coal is a chemical element with the symbol C and atomic number 6. Atomic Number number of protons in the nucleus.
The free element has a lot of uses including decoration purposes of diamonds in jewelry or black fume pigment in automobiles rims and printers ink. Light Transmission Of Carbon Element - Xgt 9000 Horiba. Biologically carbon holds a significant position and is part of all living systems.
P L is the incident power in units of W. In humans carbon makes about 185 of body mass and is the second most abundant element in the body 2. Moreover in the visible light ranging from 370 nm to 700 nm the transmission of the CNT slightly increases.
Amorphous carbon is impure carbon. The model was validated using the measured transmittances as a function of. The percentage of light transmission through clear colorless ice 7 is not greatly different from that through liquid water Fig.
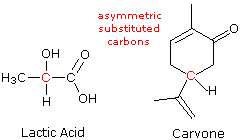
It is a heavy mobile reddish-brown liquid volatilizing readily at room temperature to a red vapor with a strong disagreeable odor resembling chlorine and having a very irritating effect on the eyes and throat. 150 years ago the natural concentration of carbon dioxide in the Earths atmosphere was 280 ppm. Are enantiomers of each other have the same physical properties except for the direction in which they rotate polarized light.
Graphite is made up of carbon. Amorphous graphite and diamond. The reduction of CO2 to Glyceraldehyde 3-Phosphate.
Title Light transmission in carbon nanotube array. On earth carbon circulates through the land ocean and atmosphere creating what is known as the Carbon Cycle. Carbon is the fourth most abundant element in the universe and is the building block of life on earth.
FE is also effective in estimating the transmission. Carbon the sixth most abundant element in the universe has been known since ancient times. Lithium hydroxide absorbs carbon dioxide from the air by forming lithium carbonate and is preferred over other alkaline hydroxides for its low weight.
Bromine is the only nonmetallic liquid element.
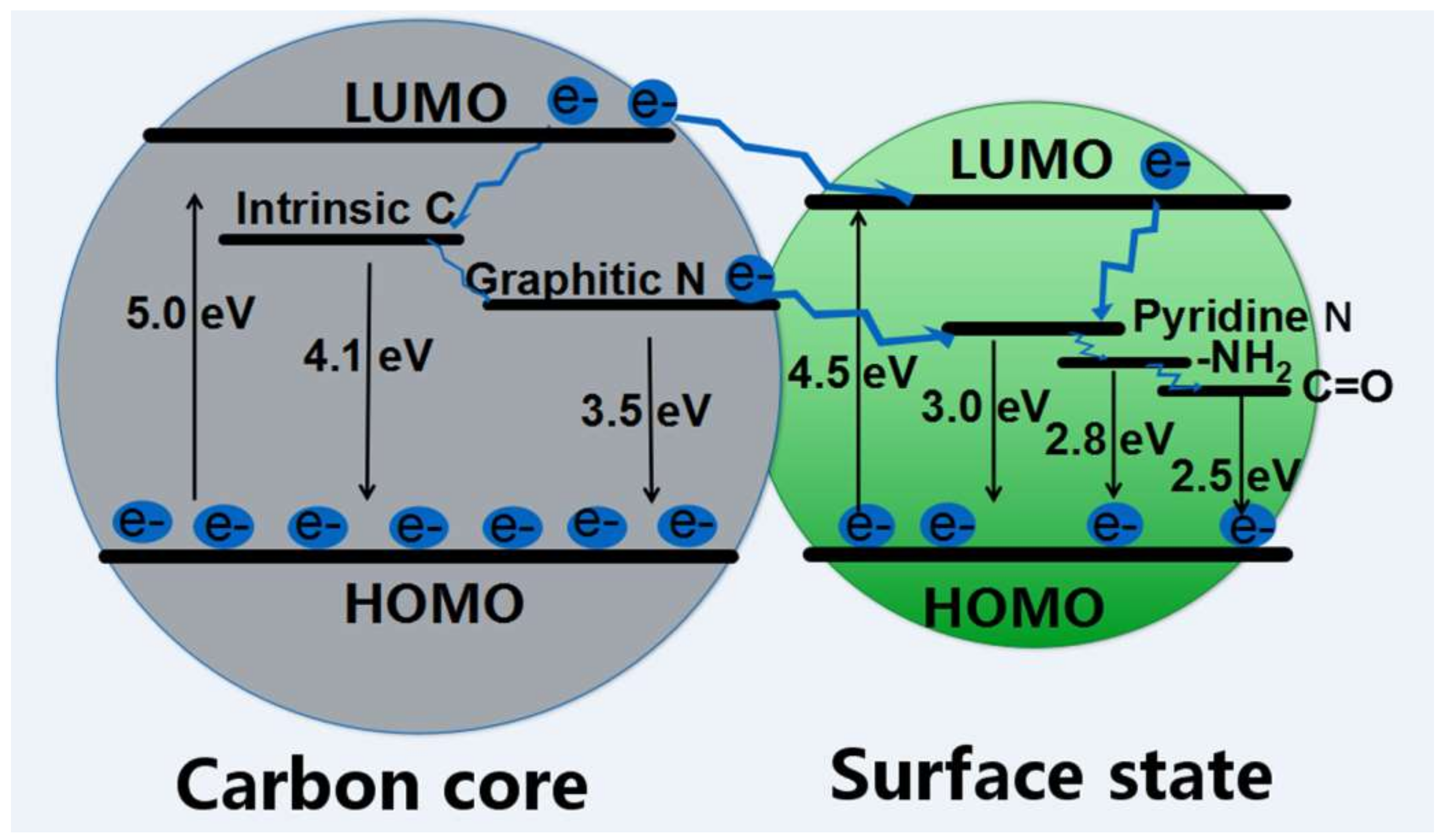
Nanomaterials Free Full Text Luminescence Mechanism Of Carbon Dots By Tailoring Functional Groups For Sensing Fe3 Ions Html

Carbon Element Information Properties And Uses Periodic Table
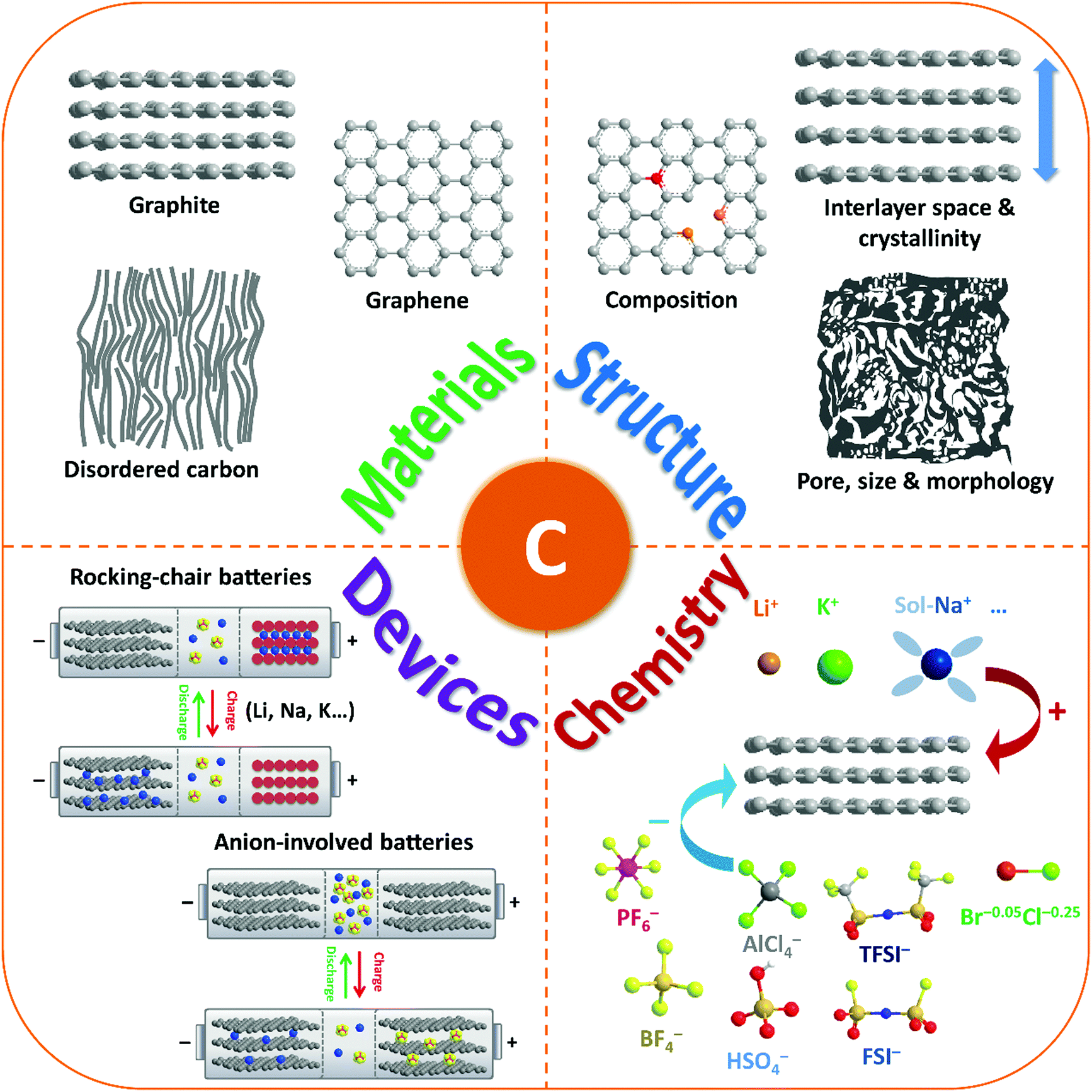
Carbon Materials For Ion Intercalation Involved Rechargeable Battery Technologies Chemical Society Reviews Rsc Publishing Doi 10 1039 D0cs00187b
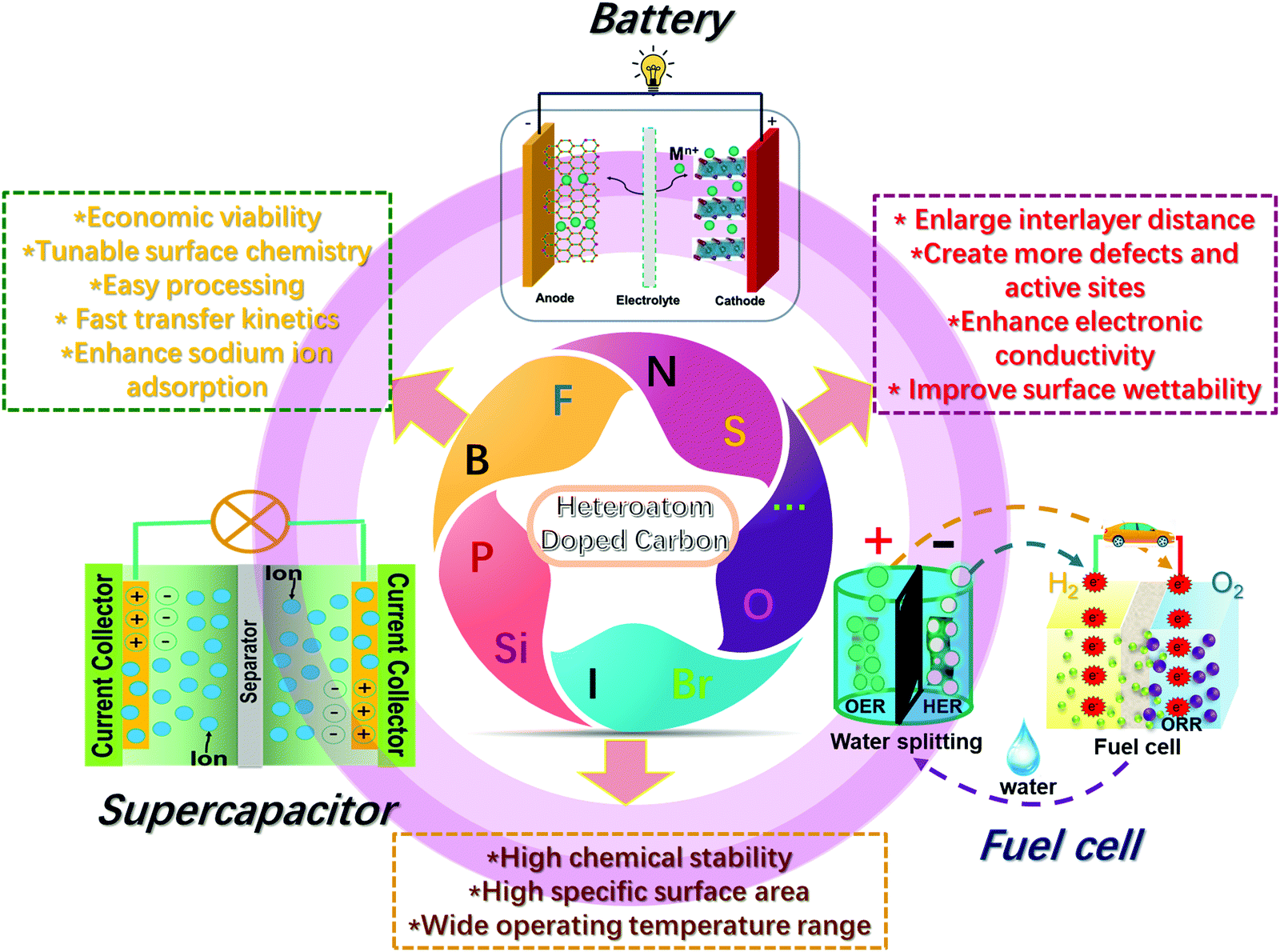
Untangling The Respective Effects Of Heteroatom Doped Carbon Materials In Batteries Supercapacitors And The Orr To Design High Performance Materials Energy Environmental Science Rsc Publishing Doi 10 1039 D1ee00166c

Introduction To Carbon Sciencedirect

Carbon Facts About An Element That Is A Key Ingredient For Life On Earth Live Science

Structures Of Diamond Comprised Of Sp3 Carbon Atoms And Of Download Scientific Diagram

Elemental Carbon An Overview Sciencedirect Topics

Electronic Configuration Diagram Vs Energy For Carbon Atom In Its A Download Scientific Diagram

Electronic Configuration Diagram Vs Energy For Carbon Atom In Its A Download Scientific Diagram
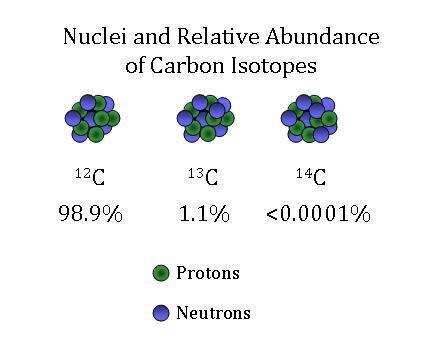
Global Monitoring Laboratory Carbon Cycle Greenhouse Gases

Structures Of Diamond Comprised Of Sp3 Carbon Atoms And Of Download Scientific Diagram

Organic Molecules Microbiology
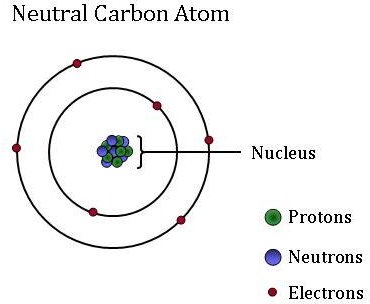
Global Monitoring Laboratory Carbon Cycle Greenhouse Gases

Electronic Configuration Diagram Vs Energy For Carbon Atom In Its A Download Scientific Diagram